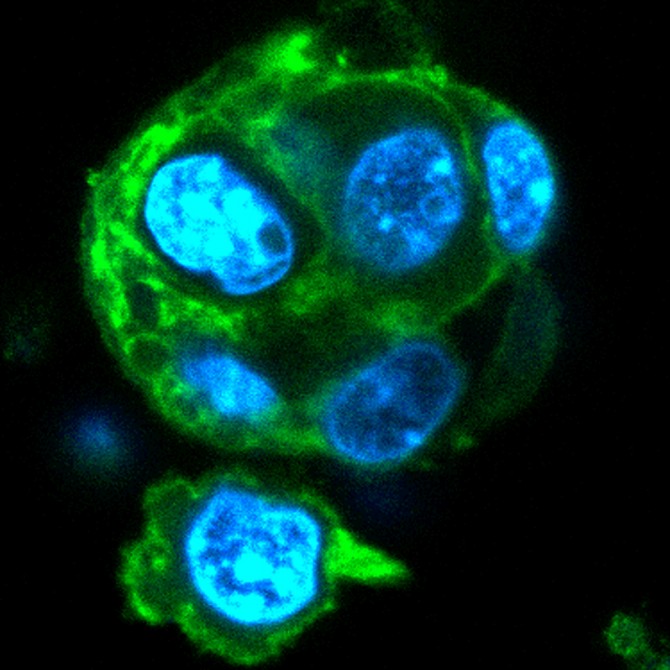
Group uses organoid to explain immune cells’ rapid response
By Tom Fleischman
In developing a swift and appropriate response to micro-organisms and vaccines, the immune system forms germinal centers that foster the massive proliferation of B cells to form target-specific antibodies.
The rapid proliferation of germinal center B cells is linked to, and required for, immune response, but there has been little direct evidence to explain how this proliferation occurs at such a rapid pace. This knowledge would not only be helpful in understanding the immune system, but also the biology of malignant lymphomas, which are spawned from aberrantly mutated germinal center B cells.
Using a tool developed in the lab of Ankur Singh, assistant professor in the schools of mechanical and biomedical engineering, Singh and Dr. Ari Melnick, the Gebroe Family Professor of Hematology/Oncology and professor of medicine at Weill Cornell Medicine, report a method for uncovering the underlying mechanism that generates B cell proliferation.
Singh and Melnick are co-senior authors of “EZH2 Enables Germinal Centre Formation Through Epigenetic Silencing of CDKN1A and an Rb-E2F1 Feedback Loop,” published Oct. 12 in Nature Communications. Lead author is Wendy Béguelin, an instructor of pharmacology in medicine in the Melnick Lab; Alberto Purwada, a recent doctoral graduate from the Singh Lab, also contributed.
Last December, Singh’s lab produced a paper detailing the use of a 3-D, biomaterials-based organoid that mimicked the early stages of a germinal center, where B cell differentiation and initiation of immunological responses take place during infection. This latest work put that research to the test to determine the reason for the intense proliferation of B cells in the germinal center.
A few aspects of this process are understood. From this massive B cell proliferation, some of the cells form strong antibodies and then undergo further differentiation into either plasma cells or memory B cells, whereas others with disadvantageous antibody gene mutations undergo cell death.
Melnick and Singh hypothesized that an “epigenetic switch” – a biochemical modification of the genome that controls conversion of normal cells into specialized B cells – was responsible for the rapid cell proliferation. Determining which switch trigged this proliferation was the challenge because of the heterogeneous nature of the germinal center B cells. Silencing gene expression in mouse models to isolate a particular protein did not answer the question, however, because germinal centers would not form in mice following gene depletion.
The organoid gave the researchers the platform necessary to re-create the germinal center, conduct mechanistic studies with specific time points and manipulation of genes or proteins, and determine exactly which biomolecule is responsible for the unrestrained proliferation of B cells.
What the team found is that a chromatin-modifying gene, EZH2, mediates germinal center formation through the silencing of a “checkpoint” gene, CDKN1A. This silencing creates high levels of a key protein, phospho-RB, which prompts a release of another protein, E2F1, which in turn further induces EZH2 expression.
This feedback loop is responsible for the germinal center B cell proliferation activity – a discovery made possible through the use of the organoid.
“This method is revolutionary,” said Melnick, who reports the competing financial interest of serving as a consultant for Epizyme, Inc. “It was next to impossible to prove that the effect of EZH2 was specifically linked to a particular phase of the cell cycle in vivo. The organoid system allowed us to address this challenge.”
The organoid yields results that are “remarkably comparable” to those from an actual immunized mouse, Singh said: “This is the first comprehensive study to show that what you get out of this [organoid] is a true germinal center programmed cell.”
This work has ramifications for cancer research, as well. The same mechanism by which EZH2 controls germinal center B cell proliferation also plays a key role in malignant lymphomas that arise from germinal center B cells. “Because of EZH2 mutations acquired during the germinal center reaction,” Singh said, “B cells undergo sustained proliferation and eventually undergo malignant transformation to become lymphomas.”
Other contributors included Martin A. Rivas, postdoc in the Melnick Lab, and Olivier Elemento, director of the Englander Institute for Precision Medicine, associate professor of physiology and biophysics and of computational genomics in computational medicine in the HRH Prince Alwaleed Bin Talal Bin Abdulaziz Alsaud Institute of Computational Biomedicine at Weill Cornell Medicine.
This work was supported by grants from the National Cancer Institute, as well as scholar awards from the American Society of Hematology and the Leukemia and Lymphoma Society.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe